Pathophysiological Concept, Current, and Previous Topics


Introduction
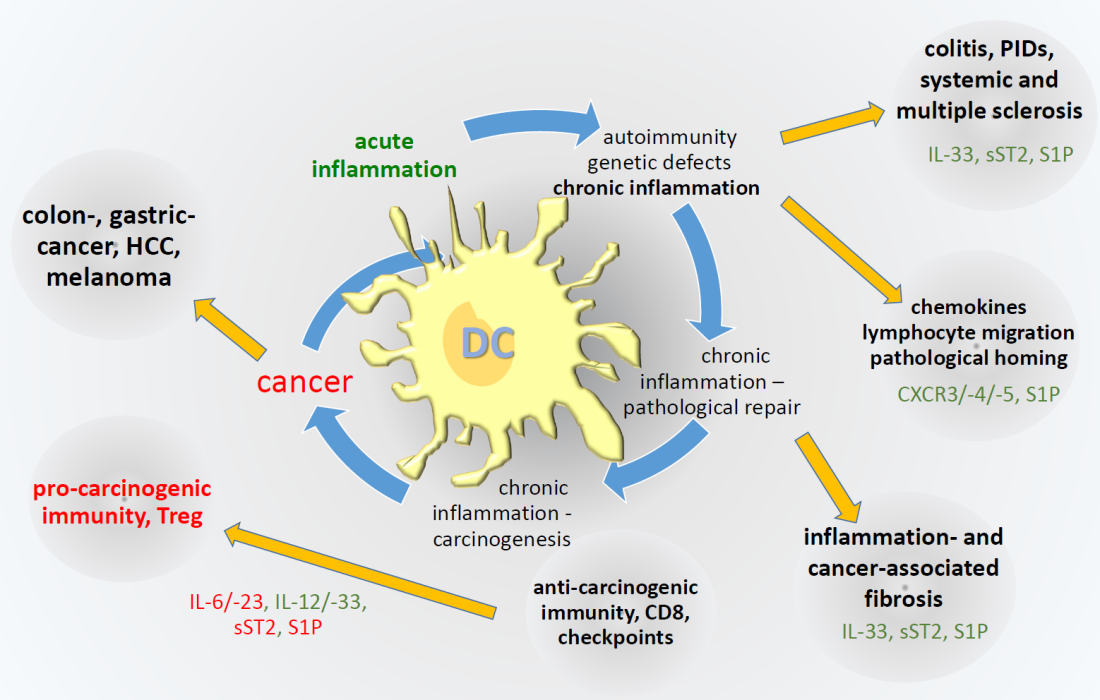
Infiltrates of mononuclear cells represent the hallmark of chronic inflammation. Following tissue injury cells of the innate and adaptive immune system invade the respective tissue through their interaction with adhesion molecules and chemotactic factors, such as bacterial components, lipids, complement fragments and chemokines. Different from acute inflammation, chronic inflammation is specifically characterized by repetitive and non-healing injury based on genetic, environmental immune deviating predisposition, and by pathological homing of immune cells finally leading to tertiary lymphoid structures as major drivers of chronicity. The interaction of the injurious agent and local cells with the professional immune system will have a decisive influence on the transition from acute to chronic inflammation, a process finally destroying vital organ function. Chronic inflammatory processes always include futile repair processes including cell protective mechanisms that eventually will support a cancerogenic transformation. Our research interest focuses on different aspects of chronicity and carcinogenesis in relevant diseases models and affected patients and the evaluation of pharmacological targets.
Current Topics
(1) Dichotomy of inflammation-associated carcinogenesis in colitis
A major discussion is going on about the dichotomy of carcinogenesis either evolving from inflammation-induced cancer or cancer-induced pro-tumor inflammation. In different projects we try to elucidate the nature of inflammation-induced carcinogenesis which can be e.g. based on a pro-tumorigenic Th2 driven inflammation or pathological crypt remodelling in the course of chronic inflammation. On the other hand, emerging tumors are able to escape the immune system or even modulate it for their own advantage. We have found, that sphingolipids, especially S1P lyase are major contributers to this dichotomy. With the help of e.g. CRISPR/Cas9 we modulate the sphingolipid-axis in hematopoietic cells or colon tissue cells and investigate the impact on disease etiology, crypt remodelling or metastasis. Furthermore, we hypothesize that the dual roles of anticarcinogenic and immunomodulatory natural products formulated in nanoemulsions may supplement cancer immune therapy.
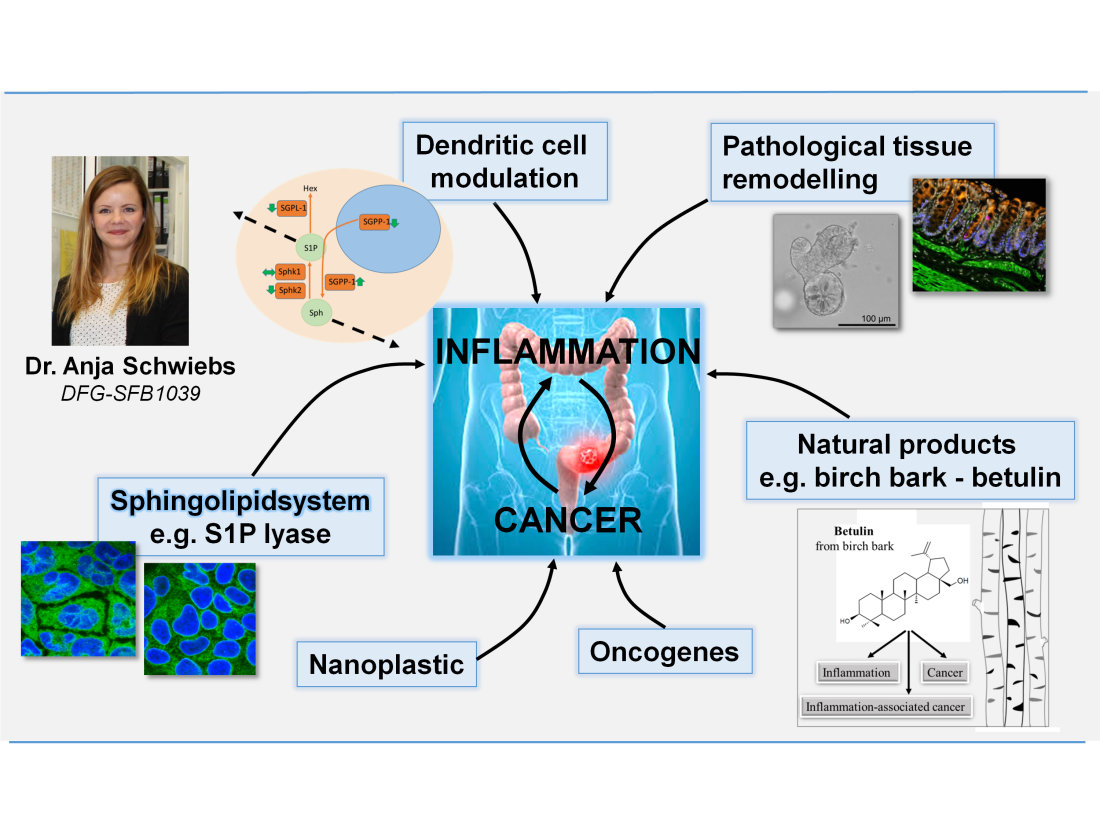
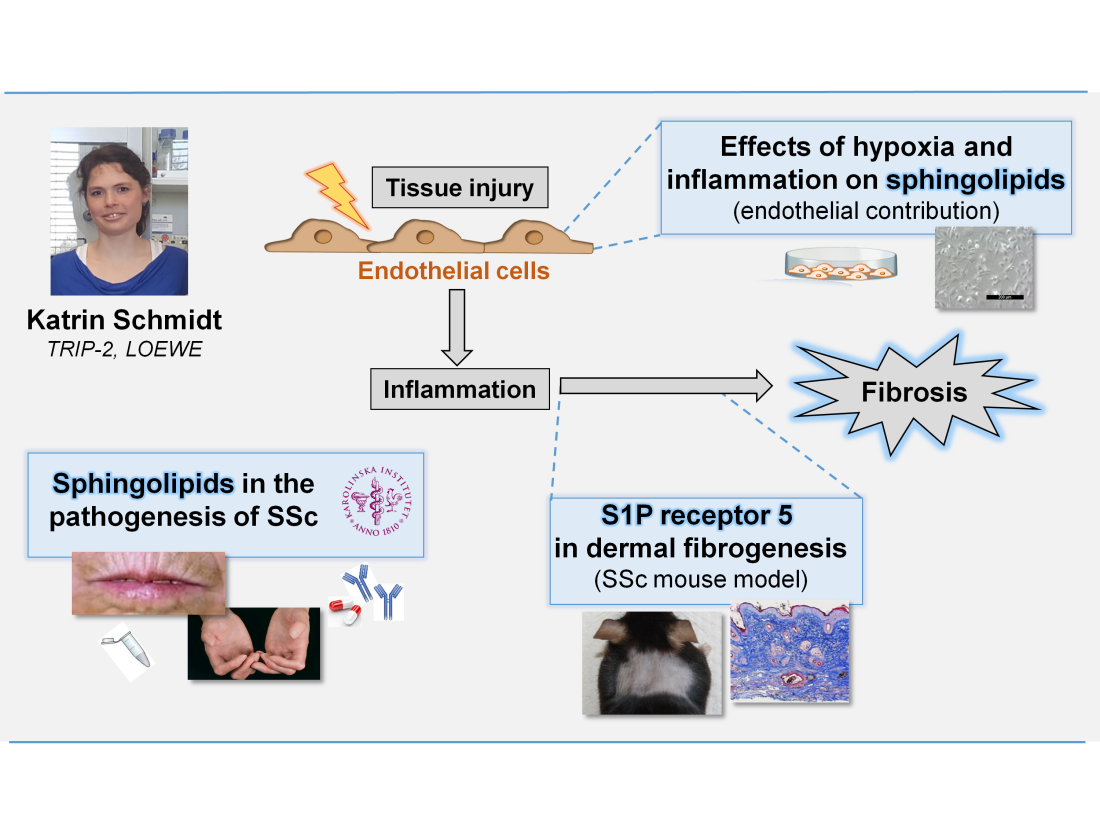
(2) Pathogenesis of Systemic Sclerosis – involvement of sphingolipid signaling
Systemic Sclerosis (SSc) is a clinically heterogeneous autoimmune and connective tissue disease affecting the skin and several inner organs. Its pathophysiology is mainly characterized by microangiopathies, inflammation and fibrosis. This project is based on the assumption that endothelial cells are major players during early vascular and dermal fibrogenesis, as well as the fact that inflammatory and fibrotic processes can be modulated by the bioactive sphingolipid sphingosine-1-phosphate (S1P) and its metabolites. With the help of in vitro and in vivo- studies, we investigate the function of S1P signaling during fibrogenesis. Supplementary, the analysis and clinical correlation of sphingolipid profiles in samples of SSc patients should contribute to a better understanding of the complex and heterogeneous disease.
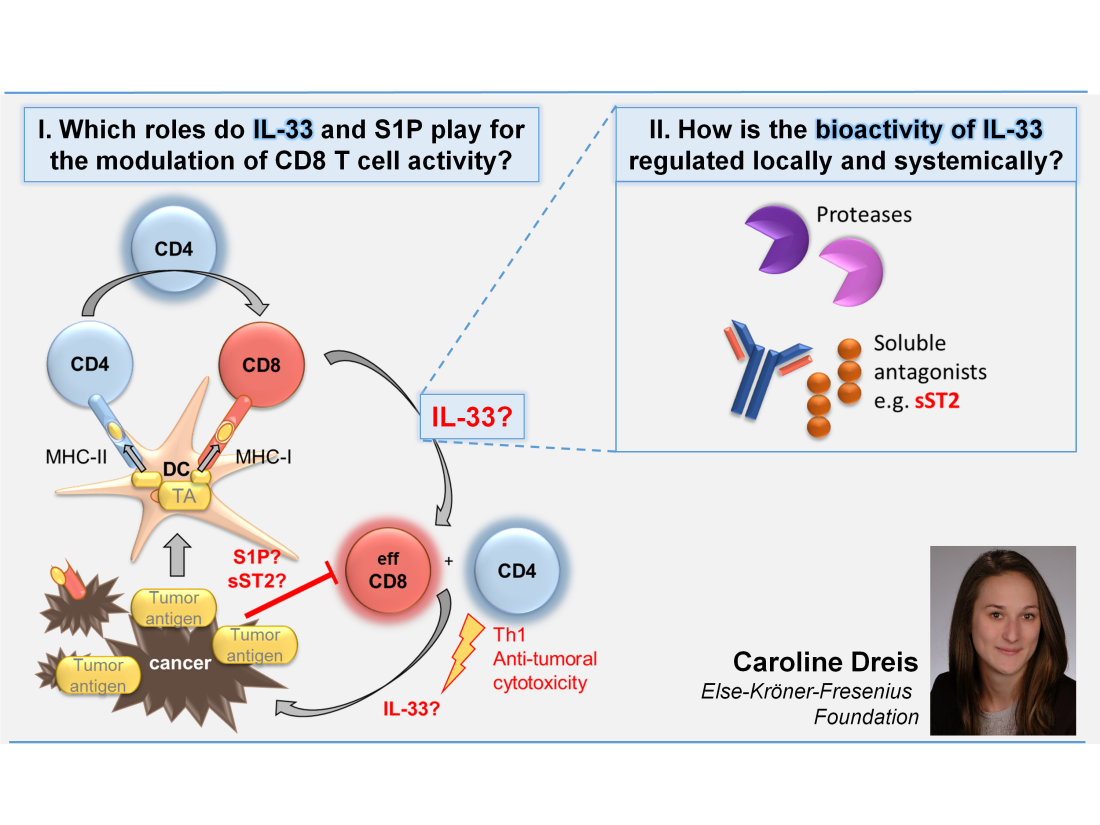
(3) Modulation of IL-1/ IL-33 TIR signaling and its role for antigen-specific cytotoxic T lymphocytes
Cancer immune therapy is based on the detailed understanding of the competition of tumor cells thriving to survive and the anticancer activity of the innate and adaptive immune system. While currently co-inhibitor blockade is in focus of clinical trials, the use of adjuvants typically acting through TLRs and signaling pathways of dendritic cells has already been used by Paul Ehrlich‘s first „cancer vaccinations“.
In this PhD project we like to follow-up on a pathway very closely related to the TLR/adjuvant, namely the TLR-like signaling pathways of IL-1, IL-33 and other IL-1 family members, called TIR. So far, investigations of pro- versus anticancer effects of IL-33 yielded contradictory results, especially considering the undefined role of the soluble IL-33 receptor sST2. While IL-33 is acting locally and very short-lived, sST2 has been attributed to cancer and fibrotic processes by us and others. Recently we have shown that IL-33 and IL-12 synergistically enhanced CD8 activity, and – introducing another modulator – that S1P may exhibit CD8 inhibitory, pro-cancer properties. Therefore, in a multicellular cytotoxicity assay setting, comprising dendritic cells, CD4, CD8 and Treg, as well as tumor antigen-bearing melanoma and colon cancer cells, we plan to analyse the regulation of IL-33 anticancer bioactivity and the influence of S1P.
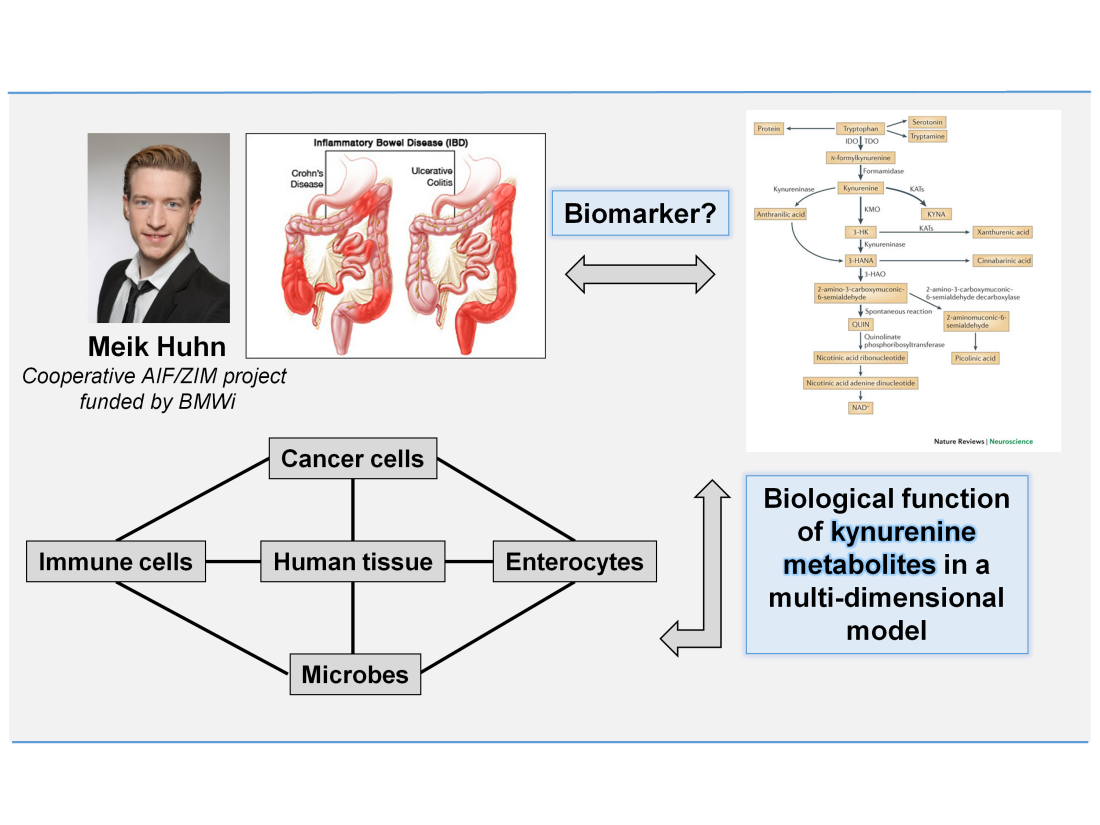
(4) Kynurenine Metabolism in Colitis – Biomarker for Inflammation and Carcinogenesis
Crohn's disease and ulcerative colitis are chronic inflammatory bowel diseases (IBD). The immune pathogenesis leads to a perpetuating mucosal inflammation based on an imbalance of pro- and anti-inflammatory cytokines. In this project the metabolic profile of the tryptophan degradation in patients with the suspected diagnosis IBD and patients before diagnosis, with relapse or in remission are compared to a healthy cohort. The project objective is to develop a biomarker matrix, e.g. tryptophan / kynurenine ratios, for a clear diagnosis, a better assessment of the stage of the disease and to control of the success of the therapy. Furthermore, more details of the biological function of additional tryptophan metabolites will be explored in a multi-cellular model. The influence of intra- and transcellular metabolism of different cell types (e.g. immune cells, enterocytes, bacterial cells and cancer cells) should be resolved in order to develop new molecular targets and therapeutic approaches.
Previous Topics
(5) Chronic inflammation and chemokines
Nadine Ogrissek, Oliver Giegold
During an immune response certain chemokines are especially important for extravasation of T cells into inflamed tissue. An upregulated inflammatory chemokine receptor signaling is known to be involved in the pathogenesis of autoimmunity. Therefore these receptors are promising targets for the treatment of chronic inflammatory disorders like type-1-diabetes, multiple sclerosis or psoriasis. However, single chemokine receptor antagonists or anti-chemokine antibodies often missed final approval for a clinic application. Our recent investigations contributed to the understanding of the underlying reasons, e.g. redundancy, systemic application and heterologous interaction of homing and inflammatory chemokines. Thus, we decided to develop a cell-based therapy for chronic inflammation with a treatment that is not only based on the effect of chemokine receptor antagonists combinations, but moreover on a localized, and “on demand“ release by means of TCR-activation-dependent antagonist expression.Our methodological arsenal encompasses conditional lentiviral vector cloning, lymphocyte transduction, FACS sorting, and dynamic flow chamber assays.
(6) S1P, sphingolipid enzymes and the Interleukin-33 - sST2 System
Florian Ottenlinger, Annika Wagner (Ranglack), Dominik Bergis, Valentin Kassis
Sphingolipids have evolved from their limited role as membrane proteins. Sphingolipid metabolites, especially the bioactive sphingosine-1-phosphate (S1P), play an important role in autoimmune diseases and inflammation. The control of the S1P concentrations with respect to phosphorylating enzymes (sphingosine kinase 1 (SphK1) and SphK2) or degrading enzymes (S1P lyase and S1P phosphatases) is of prominent interest. On the other hand dendritic cells are crucial for inflammatory responses. Therefore, one of the three sphingolipid projects is focused on the regulation of the S1P degrading enzymes, e.g. S1P lyase, in murine dendritic cells. Recently we described a modulating effect of extracellular S1P limited to dendritic cell IL-12p35 expression. Currently, we analyse the role of intracellular S1P on dendritic cell signaling using conditional sgpl1 knockout strategy and siRNA(Olga Arlt). Furthermore we are interested in interleukin-33, one of the endogenous “alarmins”. Processed differentially during apoptotic and necrotic conditions and depending on its soluble receptor it may act in a pro- or an anti-inflammatory manner. In detail we study the influence of the IL-33/ST2 and sphingolipid pathway in different human diseases:
- In patients with multiple sclerosis (MS) we are investigating the effects of FTY720 treatment in cooperation with the Clinic of Neurology. Supplementing these human studies we are working with a bioassay to determine the role of sphingolipids for IL-33 signaling (Florian Ottenlinger, Stephanie Bourdy).
- A second translational project investigates the pathophysiological mechanisms of Systemic Sclerosis (SSc) in cooperation with the Rheumatology Clinic. Assuming that endothelial cells (EC) are key players in the development of SSc in vivo we are interested in the role of EC under inflammatory and SSc-like conditions. In vivo IL-33/ST2 and several sphingolipids are analyzed in serum of SSc patients (Annika Ranglack/Wagner, see continuation above: Project 2 Katrin G. Schmidt).
- Moreover, cooperating with the Gastroenterology we study the influence of IL-33/ST2 oncancerogenesisrelated to Helicobacter Pylori (HP) gastritis. IL-33/ST2 may play a role in tumor development and survival. Therefore IL-33/ST2 is analyzed in serum and tissue samples of HP-positive patients as well as in gastric cancer patients (Dominik Bergis and Valentin Kassis).
(7) Redox regulation of chronic inflammation and carcinogenesis
Cornelia Richter, University Clinic Dresden; Martina Herrero San Juan and Heinfried H. Radeke
On the background of the simple enigma how dendritic cells may either prevent or support chronic inflammation dependent cancerogenesis we have been investigating differential TLR signaling pathways leading to either IL-12 or IL-23. Among other factors the balance between IL-12 and IL-23 modulates chronic colon inflammation and colon cancer. Based on previous mouse colitis studies we now established a colitis associated colon cancer model. Technically equipped with a mouse coloscopic, computer-aided system we are monitoring the progress of colitis and the development of tumor growth. IL-12 deficient mice develop less colitis, but are more susceptible to cancer. Additionally, in vitro we have shown that the NADPH oxidase protein p47phox down regulates IL-12 in dendritic cells. Currently we are focusing on the impact of p47phox redox and redox-independent regulation of IL-12 and IL-23 in the colitis/cancerogenesis mouse model (Cornelia Richter, Martina Herrero San Juan).
In a second project we acknowledge newly developing concepts in cancer therapy. Cytostatic agents that simultaneously exhibit immune stimulatory activity hold a great promise as a novel approach for an integrated cancer therapy. Therefore, one cooperative international project aims to investigate phytocompounds and S1P analogs regarding their antitumor activity on melanoma cells and their immune modulating effect on primary dendritic cells and T cells (Corina Danciu, Cristina Dehelean, Elisabeth Katzy, Katja Rueger, Kathrin Pfarr, Kathrin Zych; also see continuation of the project by Dr. A. Schwiebs above).
Selected Reviews and Original Publications
Schmidt KG, Herrero San Juan M, Trautmann S, Berninger L, Schwiebs A,
Ottenlinger FM, Thomas D, Zaucke F, Pfeilschifter JM, Radeke HH.
Sphingosine-1-Phosphate Receptor 5 Modulates Early-Stage Processes during Fibrogenesis in a Mouse Model of Systemic Sclerosis: A Pilot Study.
Front
Immunol. 8:1242.2017 Sep 29
Schwiebs A, Radeke H.
Immunopharmacological activity of betulin in inflammation-associated carcinogenesis.
Anticancer Agents Med Chem. doi: 10.2174/1871520617666171012124820. [Epub ahead of print] 2017 Oct 12. Review
Richter C, Herrero San Juan M, Weigmann B, Bergis D, Dauber K, Muders MH, Baretton GB, Pfeilschifter JM, Bonig H, Brenner S, Radeke HH.
Defective IL-23/IL-17 Axis Protects p47phox-/- Mice from Colon Cancer.
Front Immunol. 8:44. 2017 Jan 27
Ottenlinger FM, Mayer CA, Ferreirós N, Schreiber Y, Schwiebs A, Schmidt KG, Ackermann H, Pfeilschifter JM, Radeke HH.
Interferon-Beta Increases Plasma Ceramides of Specific Chain Length in Multiple Sclerosis Patients, Unlike Fingolimod or Natalizumab.
Front Pharmacol. 7:412. 2016 Nov 3
Bergis D, Kassis V, Radeke HH.
High plasma sST2 levels in gastric cancer and their association with metastatic disease.
Cancer Biomark. 16(1):117-25. 2016
Ottenlinger F, Schwiebs A, Pfarr K, Wagner A, Grüner S, Mayer CA, Pfeilschifter JM, Radeke HH.
Fingolimod targeting protein phosphatase 2A differently affects IL-33 induced IL-2 and IFN-γ production in CD8(+)lymphocytes.
Eur J Immunol. 46(4):941-51. 2016 Apr
Pfarr K, Danciu C, Arlt O, Neske C, Dehelean C, Pfeilschifter JM, Radeke HH.
Simultaneous and dose dependent melanoma cytotoxic and immune stimulatory activity of betulin.
PLoS One. 10(3):e0118802. 2015 Mar 10
Rüger K, Ottenlinger F, Schröder M, Zivković A, Stark H, Pfeilschifter JM, Radeke HH.
Modulation of IL-33/ST2-TIR and TLR signalling pathway by fingolimod and analogues in immune cells.
Scand J Immunol. 80(6):398-407. 2014 Dec
Arlt O, Schwiebs A, Japtok L, Rüger K, Katzy E, Kleuser B, Radeke HH.
Sphingosine-1-phosphate modulates dendritic cell function: focus on non-migratory effects in vitro and in vivo.
Cell Physiol Biochem. 34(1):27-44. 2014 Review
Giegold O, Ogrissek N, Richter C, Schröder M, Herrero San Juan M, Pfeilschifter JM, Radeke HH.
CXCL9 causes heterologous desensitization of CXCL12-mediated memory T lymphocyte activation.
J Immunol. 190(7):3696-705. 2013 Apr 1
Bergis D, Kassis V, Ranglack A, Koeberle V, Piiper A, Kronenberger B, Zeuzem S, Waidmann O, Radeke HH.
High serum levels of the Interleukin receptor soluble ST2 as a negative prognostic factor in hepatocellular carcinoma.
Trans. Onc. 6(3):311-318, 2013
Schröder M, Richter C, Juan MH, Maltusch K, Giegold O, Quintini G, Pfeilschifter JM, Huwiler A, Radeke HH.
The sphingosine kinase 1 and S1P1 axis specifically counteracts LPS-induced IL-12p70 production in immune cells of the spleen.
MolImmunol. 48(9-10):1139-1148, 2011
Richter C, Juan MH, Will J, Brandes RP, Kalinke U, Akira S, Pfeilschifter JM, Hultqvist M, Holmdahl R, Radeke HH.
Ncf1 provides a reactive oxygen species-independent negative feedback regulation of TLR9-induced IL-12p70 in murine dendritic cells.
J. Immunol. 182(7):4183-4191, 2009
Supported by
DFG funding: SFB1039, SPP1267, GRK1172; Merck KGaA; LOEWE/TRIP, Else-Kröner-Fresenius Foundation; Dr.-Hans-Kröner-Foundation; Bilateral European Erasmus Program with Romania; Faculty internal funding for Clinicians (Patenschaft) and Medical Students